What is Preclinical Research?

Preclinical research is the foundation of drug development, marking the start of the journey to identify, characterize, and refine novel therapeutic candidates. This stage involves designing and conducting studies to evaluate the efficacy, safety, and pharmacokinetics of potential treatments before they can be tested in humans. It is a long and rigorous process, typically spanning 4 to 10 years, and encompasses multiple phases, ultimately aiming to nominate a promising clinical candidate for further development.
In the pharmaceutical industry, this process is highly selective. For every research project, which may start with the screening of 200,000 to over 1 million compounds, only one or two compounds progress to become clinical candidates for human testing. Moreover, fewer than 10% of these projects has a successfully transition to a clinical candidate, as many fail during earlier phases of preclinical evaluation.
Despite its challenges, this stage is crucial for ensuring that only the most promising and well-characterized compounds progress, thereby reducing the risks of failure in later stages and safeguarding the integrity of the drug development pipeline.
WHAT ARE THE STAGES OF PRECLINICAL RESEARCH?
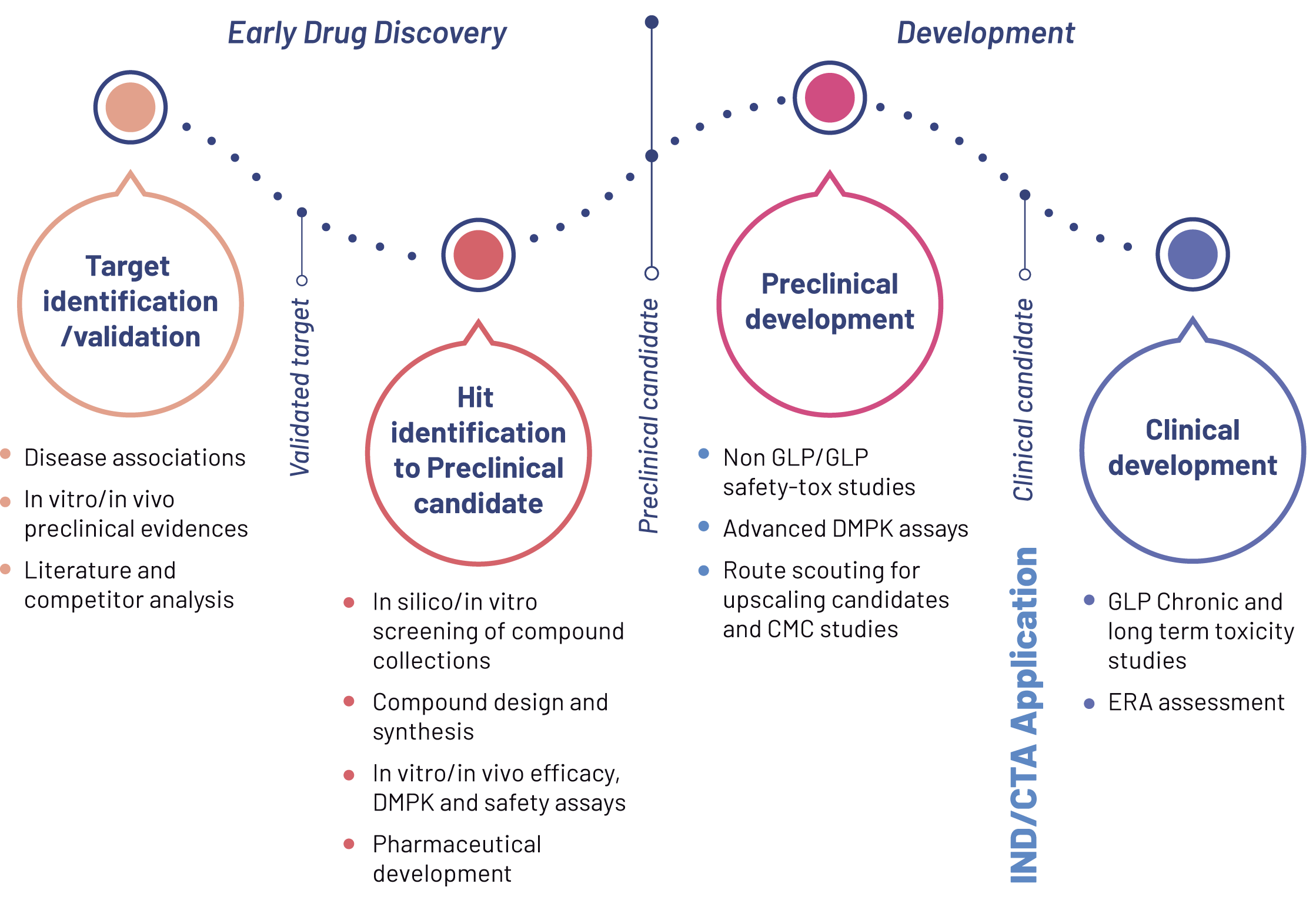
Preclinical research encompasses the early phases of drug discovery[REF1], from target identification to the nomination of a preclinical candidate and extends to later stages that include safety and toxicology studies, process scale-up, and pharmaceutical development.
EARLY DRUG DISCOVERY:
1. Target Identification and Validation
The first and most critical step in early drug discovery is identifying the optimal biological target for treating or preventing a disease. This involves determining which targets play a significant role in the disease’s pathogenesis or progression and confirming their relevance. The validation process aims to demonstrate that modulating the target (either enhancing or inhibiting its activity) results in measurable biological changes that positively impact the disease while minimizing adverse effects.
Target identification and validation rely on therapeutic area-specific strategies, underpinned by core principles such as:
- Disease association: Establishing a strong link between the target and the disease.
- Preclinical evidence: Employing in vitro models (e.g., cellular or tissue-specific assays) and in vivo systems (e.g., transgenic animal models) to confirm the target’s role.
- Literature and competitor analysis: Gaining insights from published research and understanding the competitive landscape.
A rigorous and evidence-based approach ensures that only the most promising targets progress to the next stages of drug discovery.
2. From Hit Selection to Preclinical Candidate Identification
The next phase involves identifying and optimizing molecules that can modulate the selected target's activity to achieve the desired therapeutic effect. Multiple strategies, including high-throughput screening (HTS), structure-based design, and virtual screening, are employed to discover chemical entities or biologics capable of interacting with the target. The initial molecules, termed "hit compounds”, are refined through iterative cycles of chemical optimization, supported by in vitro and in vivo testing.
Key objectives during this process include:
- Efficacy improvement: Enhancing the compound's biological activity.
- Pharmacokinetics optimization: Achieving favorable absorption, distribution, metabolism, and excretion (ADME) profiles.
- Safety profiling: Ensuring minimal toxicity and off-target effects.
- Pharmaceutical development: Addressing formulation, scalability, and manufacturability requirements.
The ultimate goal is to deliver a "preclinical candidate" with an optimal balance of efficacy, safety, and developability. Success in this stage requires a multidisciplinary and integrated approach, leveraging expertise from medicinal and computational chemistry, pharmacology, pharmacokinetics, and toxicology. Collaborative efforts across these disciplines are essential to accelerate the discovery of innovative therapeutics.
3. Preclinical and Non-Clinical Development
Once a preclinical candidate is identified, it undergoes a series of rigorous in vitro and in vivo preclinical studies to thoroughly characterize its safety profile, assess potential toxicity, and determine the safe starting dose for the first-in-human trial. These studies support the IND (Investigational New Drug) application and are part of the so-called IND-enabling package, required by regulatory authorities to ensure the safe transition from animal models to human trials under both acute and chronic exposure conditions. This package also includes manufacturing strategies and clinical protocols.
Upon IND approval by regulatory agencies, the investigational drug is authorized for administration to humans, marking the first step toward clinical investigations. The evaluation of safety continues throughout the clinical phases via non-clinical studies, aimed to assess potential chronic and long-term effects of the investigational drug.
Additionally, it is mandatory for the Marketing Authorization of Human Medicinal Products to include an Environmental Risk Assessment (ERA) aimed to identify any potential risk to the environment and ecosystem that the new drug may cause.
The overarching goal of preclinical and non-clinical studies is to establish a robust therapeutic window and to define the drug’s risk-benefit balance, ensuring that it is both effective and safe for human use.
THE REGULATORY CONTEXT [REF2]
In the early stages of drug discovery, studies conducted up to the identification of a preclinical candidate are considered non-regulated and do not need to meet specific regulatory requirements. However, as the drug progresses toward human exposure, preclinical and non-clinical studies must comply with stringent regulatory guidelines issued by national and international bodies (e.g., EMA, FDA, ICH) and adhere to Good Laboratory Practice (GLP) principles.
GLP principles govern all aspects of regulatory research, from study planning and execution to monitoring, reporting, and archiving. First established in 1978 by the FDA, GLP rules were later adopted by the OECD in the "Council Decision on the Mutual Acceptance of Data in the Assessment of Chemicals." This framework, updated in the OECD GLP Series in 1997, ensures that safety assessments are conducted under high-quality standards recognized across OECD countries.
Similarly, the International Council for Harmonisation (ICH) was established in 1990 to align regulatory requirements globally. By fostering collaboration between regulatory authorities and the pharmaceutical industry, the ICH aims to harmonize scientific and technical aspects of drug registration. Its mission is to ensure the development of safe, effective, and high-quality medicines while removing technical and regulatory barriers to promote global public health.
The application of ICH guidelines and GLP principles ensures consistency, quality, and mutual recognition of data, contributing to the protection of both human health and the environment.
IN CONCLUSION
Preclinical research is a long and complex journey that begins with basic biological studies and progresses through increasingly sophisticated investigations, culminating in and continuing during clinical trials. By integrating scientific knowledge, high-quality standards, and a harmonized regulatory framework, preclinical research supports clinical development and plays a critical role in delivering safe and effective drugs for human use.
References:
[REF1] British Journal of Pharmacology (2011) 162 1239–1249
[REF2] ICH https://www.ich.org/; EMA https://www.ema.europa.eu/en; FDA https://www.fda.gov/;
GLP OECD https://www.oecd.org/chemicalsafety/testing/oecdseriesonprinciplesofgoodlaboratorypracticeglpandcompliancemonitoring.htm
